The Anemel project recently achieved a major breakthrough in the light-like form of sustainable production of green hydrogen from low-quality water sources.
Green hydrogen has great potential for a trip to Net Zero. However, traditional production methods for this prove to be long, expensive and inefficient. Now, researchers are looking for new ways to produce green hydrogen from low-quality water sources such as seawater and wastewater.
Funded by the European Council of Innovation, Anemel Project, led by the University of Galway in Ireland, aims to develop efficient electrolizers using renewable technology. Electrolizers utilize non-critical raw materials within all basic components, including electrocatalysts and membranes. This reduces the cost of electrolyzer components, improves recyclability, reduces waste, and provides a competitive advantage.
The project is led by the University of Galway, but it has close partnerships with researchers from nine organizations across Europe. Recently, with the help of our partners, the project has reached several important milestones in our journey to achieving more cost-effective, sustainable and efficient hydrogen production. For more information, we spoke with Anemel Coordinator Dr. Pau Farràs.
Could you please explain in detail about the Anemel project and its main purpose?
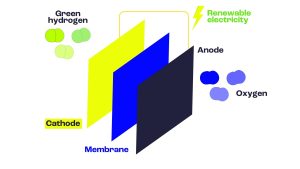
Anemel’s goal is to develop water electronics. This is an instrument that uses electricity to break down water into oxygen and hydrogen. This is an important pathway for the hydrogen economy as there are other types of technologies that can be used to obtain hydrogen, but the moisture electrode engine can be combined with renewable energy to produce hydrogen that does not emit carbon.
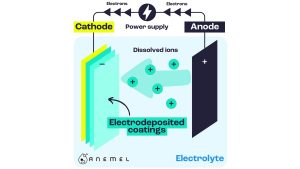
Many companies commercialize water electronics, and there are various types depending on the operation and its components. Water Electro Lither is not new, and some operations date back to 100 years. One of the main problems with water electronic agents is the use of strong bases such as potassium hydroxide and sodium hydroxide at high corrosive and dangerous concentrations. Therefore, building large plants becomes a serious health and safety concern. Conversely, new polymer electrolyte membrane (PEM) electrolithers require ultra-pure water to run. In both cases, water must be purified to the highest possible level to ensure stable electrolysis.
Anemel aims to develop components that enter the electrodes (electrodes and membranes) that are compatible with impurities in water. We do this through a step-by-step approach of increasing the challenging aspects of operation.
Typically available anion exchange membrane (AEM) electrolithers work using concentrated solutions of potassium hydroxide. This isn’t as bad as the old alkaline electrolysis, but it’s something to avoid. At Anemel, the first challenge is to reduce the concentration of potassium hydroxide, reducing the risk and corrosion of electrical agents. This makes it easy to operate and inexpensive.
The second stage is operating the electrolyzer under the same conditions as the PEM electrolyzer. The problem with PEM electrolither is that it requires the use of precious metals such as iridium, ruthenium and platinum, which are very expensive. To achieve large-scale hydrogen production, many of these precious metals need to be used, which are not very abundant. Anion exchange membranes can use cheaper and richer metals, but they must be constructed in a way that can be used under ultrapure water conditions.
The final step is to work with impure water. In our case, the way to define impure water is to add salt. We have a salt concentration that is very similar to marine salt. The idea is that many impure streams, or water streams, that do not compete with drinking water (sea, sea, wastewater, etc.), contain salt. I would like to demonstrate that this electrolyzer can be operated under salty conditions.
There are specific reasons why I chose to focus on improving the AEM electrolither over PEM. The salt is sodium chloride, and when the electrolyte in the water contains chloride, the reaction of the oxidized water molecules competes with the oxidation of chloride ions to chlorine. This is a corrosive gas that destroys the components of electronic agents. To avoid this reaction and minimize the risk, you should essentially operate from pH neutral. If you want to operate the PEM electrolither under acidic conditions, the difference between chloride ions and oxidized water is very small. This doesn’t have much time to do surgery. Because if you make a mistake or degrade the catalyst to some extent, it will oxidize the chloride to chlorine gas very easily instead of the reaction you want to occur. This is why it works under these AEM conditions, in contrast to PEM.
In summary, we want to develop an electrolizer that is more robust than the electrolizers available in the market today. The only way to achieve clean hydrogen production prices that compete with hydrogen produced using fossil fuels is to develop highly robust and long-lasting electrolyzers. Furthermore, it is important to develop components that are less susceptible to decomposition to extend the lifespan of these technologies.
Can you highlight recent results from the project?
From an electrolizer perspective, our main objective is to develop an electrolither with 1kW of power. To prepare a 1kW electrolither producing sufficient green hydrogen, various parts of the electroliser had to be designed to achieve this with very high efficiency. To do this, partners at Ecole Polytechnic Federare de Lausanne (EPFL) in Switzerland were able to design a 1kW unit and use simulations to find the right configuration to optimize the operation to have very good efficiency.
The EPFL team simulated a 1 x 1 cm² membrane electrode assembly (MEA) based on commercially available materials. MEAs are formed in layers, like sandwiches. The center is a membrane, with catalyst covered on both sides. The membrane separates the hydrogen and oxygen gas produced during the electrolysis process. Catalysts accelerate electrochemical reactions. Each side reaction requires a different material unique to the evolution of hydrogen and oxygen.
The researchers created this computational model taking into account certain simple parameters such as membrane thickness and temperature. Among other things, the team found that PH is the most influential parameter in the performance of these electrolizers. The results of the simulated membrane electrode assembly studies were published in a peer-reviewed paper.
Also, advances have been made in relation to the stack elements of the electrolizer. The stack consists of different cells, and for these electrolithers, five cells create the stack. Multiple cell modules allow you to generate more hydrogen by adding more cells or stacks. To examine the components that enter each cell, instead of looking at the entire stack, you can use only one cell to examine its behavior and components. This was done by operating a single-cell system under salty conditions alongside a partner researcher from Tu Berlin in Germany and an EPFL in Switzerland.
In another important outcome, the EPFL group led by Professor Xile Hu was able to demonstrate the operation of anion exchange membrane electron membrane (AEMWE) systems using pure water at extremely high current densities, resulting in greater hydrogen production. This is one of the main advantages over 100-year-old technology that operates at very small current densities. This means that for the same amount of hydrogen produced, there is a much larger amount and a field of technology. This study, published in Angewandte Chemie, demonstrated AEMWES operating at ultra-high current density of over 800 hours, without degradation in performance. In comparison, cutting-edge benchmark electrolithers maintain such current density for only a few seconds. This represents an impressive increase of 500,000 times the performance.
Our team is also working on electrocatalyst design. This is a component that sits along with the membrane. The electrodes in the AEMWE stack represent a significant share of overall electrolyser costs. Therefore, many studies have advanced to the development of new, efficient and cost-effective catalysts for electrodes that may work well in alkaline environments, leading to reduced costs and improved performance.
What are your top priorities for the future?
So far, we have mainly operated at lower hydroxide concentrations than traditional AEMWE. The priority for the rest of the project is to use pure water and salt water to work the stack. It focuses on continuing electrocatalyst and membrane development so that it can operate under these conditions.
Additionally, if you move from a single cell to the stack level, you need to start generating more amounts. So we must now expand the most active catalysts and best membranes we are doing now. Instead of producing a Mg-scale catalyst and a cm² scale membrane, you need to go through a few grams of catalyst and a square meter membrane. This scale-up process requires thorough research and development, allowing you to scale up the process and conform to the circularity of the various components you want to generate.
How important is collaboration in your work?
Collaboration is important as you saw, as you saw, there are different components and it is impossible for one group or organization to deal with everything. At electrolyzer manufacturers, most of the time, expertise lies in assembling components purchased from other locations. They don’t develop everything from scratch because they require a wide range of skills and know-how. Collaborating with different experts from different institutions is the only way this can be achieved.
Partnerships with the project’s companies are extremely beneficial when it comes to scaling up due to the expertise gained from expanding previous products. It is essential that their opinions on how we should expand and help bridge the gap between academia and industry.
This article will also be featured in the 22nd edition of Quarterly Publication.
Source link

