Texas-based Neurotech startup paradromics announced on Wednesday it has secured strategic investments from Saudi Arabia’s Neom. As part of the transaction, Paladromics will establish the Regional Brain Computer Interface (BCI) Center of Excellence.
The investment was led by Neom Investment Fund, Neom’s strategic investment arm. Paradromics did not disclose any related funds.
“This partnership marks a pivotal moment for Paradromics and the broader BCI industry,” said Matt Angle, founder and CEO of Paradromics, in a statement. “Both Neom and Paradromics have a vast vision for a highly aligned mental health future. Together, we will accelerate BCI innovation rates and become an impactful BCI-based treatment. You can increase access to the site.
With this investment, Paradromics is joining the growing list of Neurotech startups that will challenge Elon Musk’s Neuralink. Another startup is Synchron, a New York-based brain interface company supported by Bill Gates and Jeff Bezos.
Paradromics develops brain computer interfaces. This is a system that reads brain signals and converts them into commands from external devices. The startup will work with Neom to promote BCI-based treatments and set up what is called “premier centres for BCI-based healthcare” in the Middle East and North Africa.
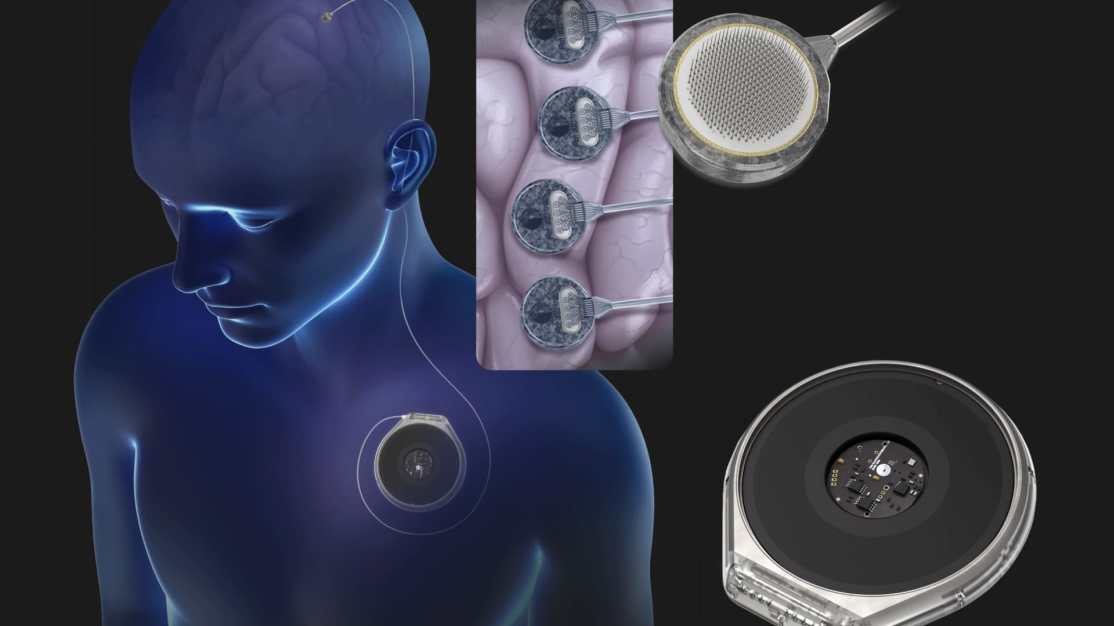
Paradromics is one of several companies in the race to bring BCIS to the market, along with celebrities like Elon Musk’s Neuralink. Earlier this month, Neuralink shared that it had implanted the technology in three human patients. Other players in the space include precision neuroscience and syncron (the latter supported by Jeff Bezos and Bill Gates).
So far, none of these companies have received final approval from the US Food and Drug Administration (FDA).
Paradromics’ proprietary BCI, Connexus Direct Data Interface, uses an array of small electrodes implanted directly into brain tissue. The purpose of this technology is to help patients with severe paralysis recover their communication skills by deciphering neural signals.
The company is preparing to begin its first human trial later this year, and began official patient registration in July. Although it has not yet secured FDA approval, Paradromics received the FDA’s breakthrough device designation in 2023, indicating that its technology is committed to addressing unmet medical needs .
Neom, located in northwestern Saudi Arabia, was announced in 2017 by Crown Prince Mohammad bin Salman. The Neom Project has acquired the status of innovation hubs, envisaging futuristic regions featuring floating industrial complexes, global trade hubs, luxury tourist resorts and linear cities running completely with renewable energy. It’s there.

Source link

