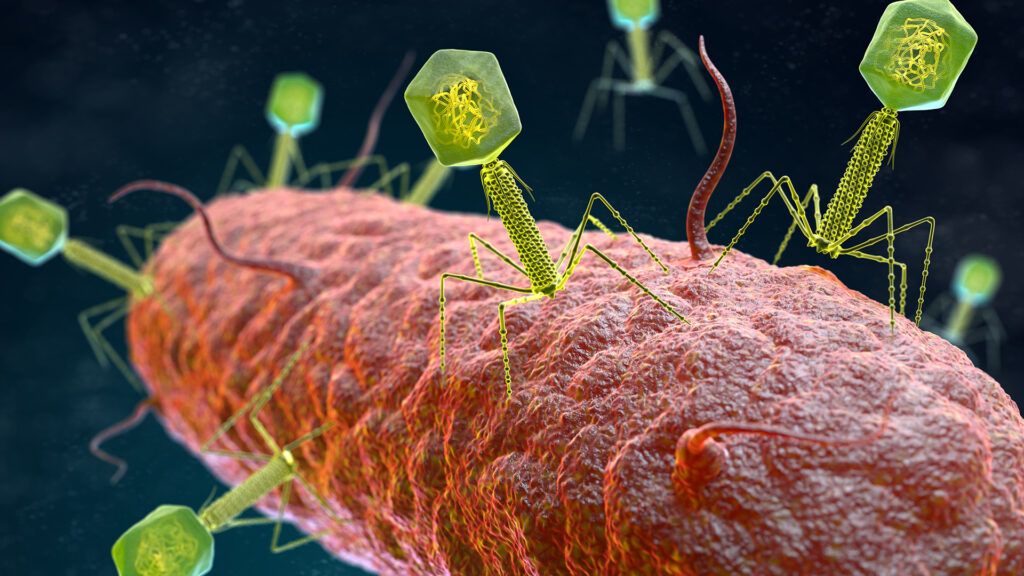
As the efficacy of antibiotics decreases, clinical trials are increasing. Cellexus is committed to supporting the development of phage therapy that uses bacteriophages to combat superbugs.
Antibiotics, including antibiotics, antivirals, antifungals and antiparasix are drugs used to prevent and treat infections in humans, animals and plants.
So-called superbugs are microorganisms, usually bacteria, that are resistant to one or more antibacterial agents previously used to treat them.
For almost 100 years, antibiotics have helped animals and humans live longer and healthier lives. The widespread and frequently inappropriate use of antibiotics has promoted significant and increased cases of multidrug resistant bacteria (AMR) overall.
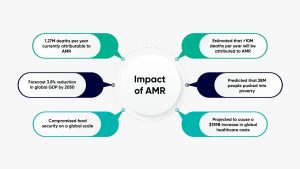
It is estimated that 4.71 million deaths were associated with bacterial AMR in 2021. This includes 1.14 million deaths caused by bacterial AMR. By 2050 each year, ² has been presented as a major cause of human mortality.
The threat of AMR is not limited to human health, but it should be viewed in a broader, healthier context in which AMR affects the health and productivity of animals and crops. The impact of AMR is projected to cost 1tr by 2030 and by 2050 the world will lose 3.8% of GDP.
In 2015, the World Health Organization (WHO) committed to the Global Action Plan (GAP) to tackle AMR. From there, as of November 2023, 178 countries developed AMR National Action Plans (NAPS) in line with GAP. A key element of the plan is the development of new antibiotics, in which bacteriophages (also known as phages) are included as a highly promising solution.
What is phage?
Phages are viruses that target and kill bacteria, and are the most abundant organisms on the planet. Phages can be found in all natural environments, from human intestines to Antarctic soils. To put this in context, it is estimated that there are 1031 phage particles in the world with 200 million tons of biomass.
Each phage that evolves along with a bacteria is usually special to target a specific bacterial strain, and for each bacteria there are multiple phages that hunt it.
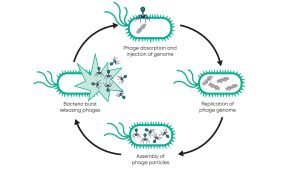
Phages hunt bacteria because they must replicate using bacterial cell machines. When the phage finds the target bacteria, it binds to the surface and injects the genetic material into the bacterial cells. It then hijacks the bacterial cell machine to create more phage particles that form until the bacteria burst, releasing a large number of phages to go hunting.
Phage therapy and challenge status
Despite being discovered and used as a therapeutic long before the discovery of antibiotics, phage therapy was largely discounted when antibiotics became available. Antibiotics outperformed phage therapy because they provided widespread infectious efficacy against a wide range of bacteria and were inexpensive and easily administered.
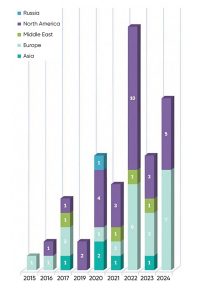
Now that antibiotics are losing effectiveness, the eyes are back to phage therapy as a solution. High-throughput laboratory techniques such as genetic sequencing and advanced bioinformatics are readily available, making preparation of safe and effective phage therapies easier to achieve. Over the past decade, the number of phage-based clinical trials has increased significantly, indicating a renewed interest in phages.
Nevertheless, many challenges remain, requiring coordinated efforts from a variety of stakeholders, not just researchers and clinicians, but also funders, regulatory agencies and governments. .
The development of tools to streamline phage isolation, analysis and production is essential for the broad implementation of phage therapy. At Cellexus, we are committed to providing phage scientists with the perfect cultural platform to overcome the unique challenge of producing phages and supporting the critical work of fighting AMR.
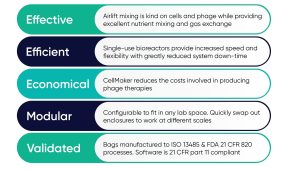
CellMaker systems offer outstanding performance and process benefits, focusing on three key aspects: resource utilization, regulatory compliance, and reliability.
resource
CellMaker is designed to make the most of the resources available (time, space, money).
Cell makers are ideal solutions for producing phages at very high titers (concentrations) and are 10-100 times higher and more frequently than can be achieved using other types of bioreactors. . The ability to generate phages with much higher titers reduces production costs by using smaller culture volumes. Additionally, generating phages at higher concentrations will remove and/or reduce costly and time-consuming downstream processing steps.
Higher yields provide highly effective gas and nutrient exchange to support bacterial growth, but mechanically associated with traditional bioreactors that can damage cells and phages as well. This is due to unique air-fuel mixing technology that does not have the power.
Patented cell manufacturer airborne bioreactors are single use and actually eliminate the risk of cross-contamination during cell culture processes and reduce the burden of process verification.
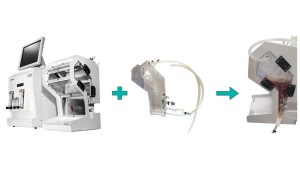
Cell maker single-use bioreactors maximize laboratory productivity by minimizing turnaround times for bioreactors. A virtually turnkey solution, cell makers can be used in minutes and can disable cleaning, assembly and sterilization processes associated with reusable bioreactors, making them more sustainable It will be the solution.
In addition to time and money, space is usually a limiting factor within a cell culture suite. CellMaker is a small modular system that can be configured to fit in virtually any laboratory. A single controller allows you to control one or two 8L and/or 50L enclosures, providing scalability of 1.5L to 100L, requiring minimal lab space.
Regulations
Made from an ISO Class VII Cleanroom with ISO 13485:2016 verified and FDA 21 CFR 820 Compliant, the cell maker bioreactor bag provides the regulatory compliance required for rapid translation from research to therapeutic production. The ability to rapidly initiate the production of therapeutic phages is essential to effectively responding to the development of antibiotic-resistant pathogens.
Supporting the system’s operation is intuitive and highly usable cell maker software that provides complete control and cutting of the cell culture process. CellMaker software can operate in research mode or GMP manufacturing mode according to 21 CFR 11 Part 11.
Reliability
Cellmaker sets a new standard for bioreactor reliability. Repeated assembly and disassembly of a bioreactor is not only expensive, but can lead to container damage, seal failures, and process problems. CellMaker removes all sources of these potential batch failures. With few moving parts and no requirements for assembly or maintenance of the vessel, cell manufacturers are always ready to use. All cell manufacturers’ bioreactor bags are rigorously inspected and pressure tested after production, so you can rest assured that the cell manufacturer will not disappoint you.
Lead the way
Cellexus is at the forefront of phage production technology and is developing a cell manufacturer platform to further support the rapidly evolving requirements of phage scientists. We always want to discuss specific requirements and work with our partners to overcome specific challenges. If you’d like to explore how cell makers can help you with your workflow, feel free to contact us.
reference
GBD 2021 antibiotic resistance collaborator. Global burden of bacteria antibiotic resistance 1990-2021: Systematic analysis with forecasts up to 2050. Lancet. 2024 Sep 28; 404 (10459): 1199-1226. doi: 10.1016/s0140-6736 (24) 01867-1. EPUB 2024 Sep16. PMID: 39299261; PMCID: PMC11718157. O’Neill, J. (2014) Antibiotic Resistance: An Attack on Crisis for National Health and Wealth. Antibacterial resistance review, 20, 1-16. World Bank – Jonas, A.B.; Irwin, A. ; Berthe, F., Cesar, J., Le Gall, F. G.; Marquez, p.v. (2017). Drug-resistant infections: Threats to our economic future (Volume 2): Final Report. HNP/Agriculture Global Antibacterial Resistance Initiative Washington, DC: World Bank Group. Chevallereaau, A., Pons, B.J., Van Houte, S. et al. (2022). Interactions between bacteria and phage communities in the natural environment. Nat Rev Microbiol 20, 49–62. https://doi.org/10.1038/S41579-021-00602-Y Strathdee, SA, Hatfull, GF, Mutalik, VK, Schooley, Rt. (2023) Phage therapy: From biological mechanisms to future directions. cell. January 5, 2023; 186(1): 17-31. doi: 10.1016/j.cell.2022.11.017. PMID: 36608652; PMCID: PMC9827498 Whitford, WG, Petrich, MA, Flanagan, WP. (2019). Environmental Impact of Single Use Systems, Disposable Techniques in Biopharmaceutical Manufacture, 2nd Edition, John Wiley & Sons, Inc.
This article will also be featured in the 21st edition of Quarterly Publication.
Source link