simple facts
Milestone: Scientists develop chemical recipe to observe biological molecules
Date: October 23, 2007
Location: University of California, Berkeley and other laboratories
Who: A team of scientists led by Carolyn Bertozzi
In 2007, scientists published a paper describing a new type of biochemical recipe. This method allows scientists to see what is happening inside an organism in real time.
Glycans are one of the three major classes of biomolecules (along with proteins and nucleic acids) and are thought to be involved in inflammation and disease, but scientists have always found them difficult to visualize. To do so, Bertozzi built on a chemical approach pioneered by biochemists K. Barry Sharpless of the Scripps Research Institute and Morten Meldal of the University of Copenhagen.
you may like
Sharpless laid out his vision for “click chemistry,” a way to quickly build complex biomolecules by snapping together small subunits.
Biological molecules often have a backbone of bonded carbon atoms, but the carbon atoms do not want to bond. Historically, this meant that chemists had to use painstaking, multi-step processes that used multiple enzymes and left unwanted byproducts. While this was fine for labs, it was a disadvantage for mass producing biomolecules for pharmaceutical use.

Sharpless realized that if he could combine simple molecules that already had complete carbon frames, he could simplify and scale up the process. All I needed was a fast, strong and reliable connector.
Separately, Sharpless and Meldal encountered an important connection point: a chemical reaction between compounds called azides and alkynes. The trick was adding copper as a catalyst.
The reaction was very strong and rapid, occurring more than 99.9% of the time without producing any by-products.
But for Bertozzi, there was a problem. Copper is highly toxic to cells.
So Bertozzi scoured the literature and devised a safe click chemistry inside living cells. She found the answer in research decades ago. The idea is that if the alkyne were forced to form a ring, the azide and alkyne would react “explosively” without the need for a catalyst.
you may like
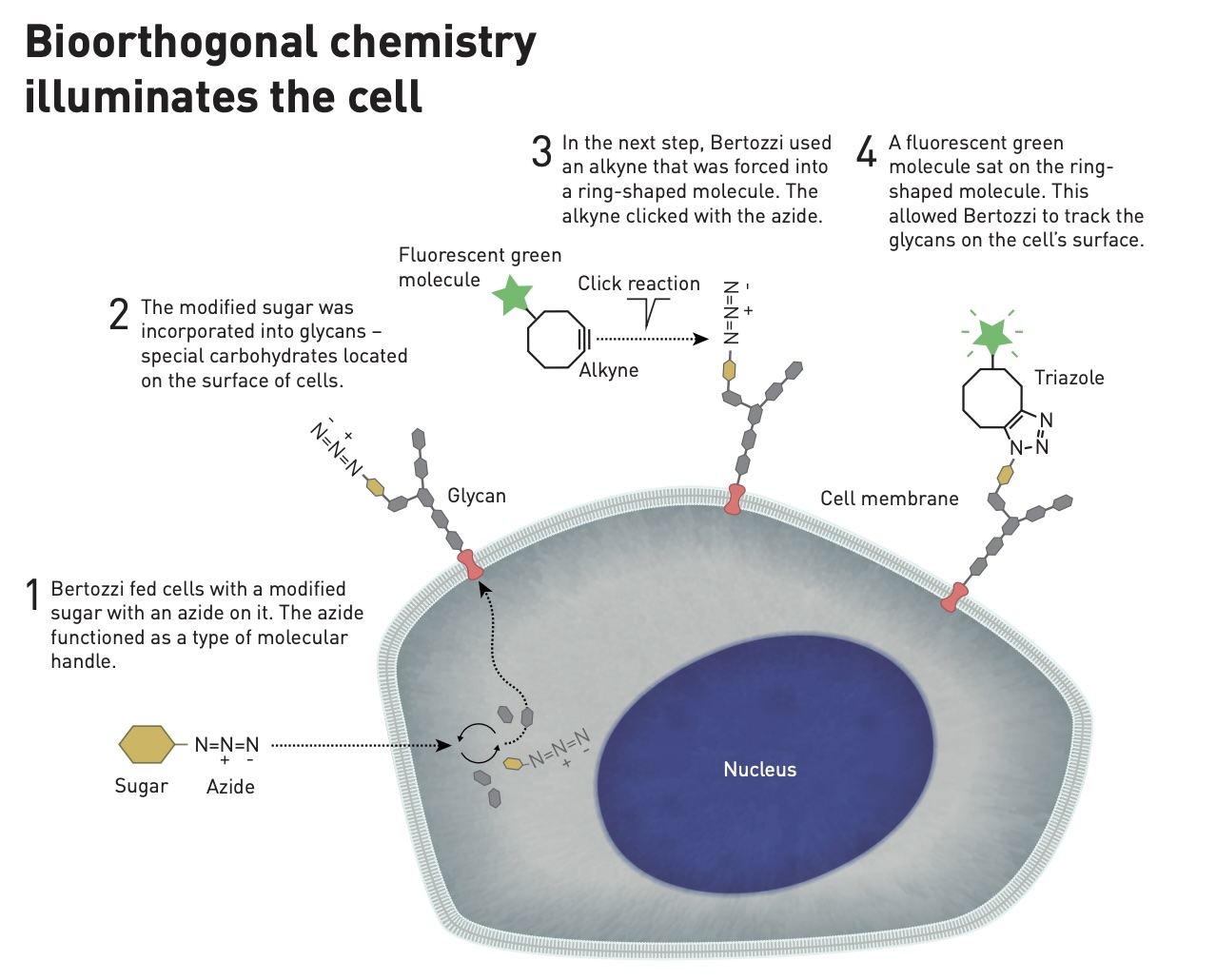
In 2004, her team demonstrated that this reaction could be used to attach azide molecules to living cells without harming them. Then, in 2007, Bertozzi and colleagues used her method to visualize glycans in living hamster cells.
Her process involved incorporating azide-modified carbohydrate molecules into glycans within living cells. When they added a cyclic alkyne molecule attached to a green fluorescent protein, the azide and alkyne clicked together, and the green-glowing protein revealed the location of the glycan within the cell.
Bertozzi termed this process “bioorthogonal” click chemistry. It is so named because it is orthogonal to, or does not interfere with, the biological processes that occur within cells. Her research has proven important in understanding how small molecules move within living cells. It has been used to track glycans in zebrafish embryos, to see how cancer cells are safe from immune attack using sugar molecules, and to develop radioactive “tracers” for biomedical imaging. And click chemistry has accelerated the drug discovery process more broadly.
In 2022, Sharpless, Meldal, and Bertozzi were awarded the Nobel Prize in Chemistry for their work on click chemistry.
Source link