The 8-year-old fell rapidly in just a few months. In August 2023 he was playing football running, but by September, unwilling muscle contractions had both his ankles. By October, he had lost the ability to run and play sports, and by late November, he had collapsed frequently, so the physical therapist advised him to get a wheelchair.
Genetic testing confirmed the cause of sudden deterioration in children. He carried two mutant copies of the gene called HPDL. HPDL proteins help to create an antioxidant called coenzyme Q10, which is important for the function of mitochondria, the cell’s powerhouse.
HDPL defects are rare and may vary in severity depending on which mutation a person carries. In the boy’s case, the telltale symptoms of muscle spasticity and paralysis did not appear until his eighth birthday, but two of his siblings died in a more serious childhood, characterized by brain damage and seizures.
You might like it
The children’s parents feared that he might meet the same fate given that there was no treatment available.
“When we met, they were very scared,” said Dr. Claire Miller, a pediatric neurologist who specializes in motor disorders at NYU Langone Health. At the time, in November 2023, the child was unable to pass the hospital lobby without falling, and his condition deteriorated significantly from the week to the next week.
However, with the help of Miller, another NYU expert, with the special permission of the Food and Drug Administration, the child began experimental treatment for his condition, which had never been tested in humans before. The treatment contains molecules that are likely to work by helping the body bypass the lack of HDPL to make CoQ10, researchers believe.
Related: Scientists Discover Single Molecules That Could Treat Rare and Catastrophic Mitochondrial Diseases
Within a month, the boy was able to walk more than half a mile (1 kilometre) through Central Park with his family. Fast forward to today, he joins them on a 4-mile (6 km) hike, and also controls the go-kart pedals himself. His strength is coming back with his energy and stamina.
“Lower improvements (such as walking easier, having more energy) are hopeful that they remain anonymous, his family told Live Science via email. “It’s reassuring to know that treatment is making a difference.”
For now, it is for children to continue taking oral medications every day, and so far, regimens are not about side effects. Children’s healthcare teams hope to test treatments in a wider group of relevant patients with these HPDL deficiency, and subsequently affect COQ10.
“For us, attempting experimental treatment gave children a better chance of living,” the family told Live Science. “And we’re grateful for the opportunity.”
From mouse to human
This intrahuman clinical trial was enabled by promising laboratory experiments in lab mice in the NYU Grossmann School of Medicine and the Department of Radiation Oncology at Perlmutter Cancer Center. Some of these mouse experiments are described in a new paper published on Wednesday (July 9) in Nature, with details of one patient trial.
Before their latest work, Pacold Lab published research that helped unlock one of the roles of HPDL in Mitochondria. They found that proteins meet the first step in the chain reaction that involves the creation of antioxidant COQ10. In summary, HPDL is used to turn a compound called 4-HMA into another compound called 4-HB and build a “head” of CoQ10.
CoQ10 is thought to help mitochondria that otherwise damage them to help expand diffusion-reactive molecules, which may also be important for the mitochondria fuel-making process, Pakord told Live Science. Also, when other functions are performed in the body, especially at cell membranes, and people are not making enough money, their defects can cause widespread problems, especially in energy-hungry organs such as the brain, kidneys and muscles.
HPDL defects also fall into the wider umbrella of Coq10 defects, as proteins help to make this antioxidant head.
COQ10 can be used as a supplement, but when taken in this way, it rarely enters the brain. The reason is not fully understood, but because antioxidants are known to not be absorbed by the intestines, it is less in the bloodstream, and even fewer molecules are larger, so it is less likely to cross the brain’s protective barrier, says Pacold. It may also be hydrophobic or greasy, which could possibly interfere with movement within the body, he added.
For these reasons, scientists are looking for other ways to increase levels of antioxidants in the central nervous system and treat these patients. The pathway defined by Pacordo and his colleagues provided clues as to how to do it.
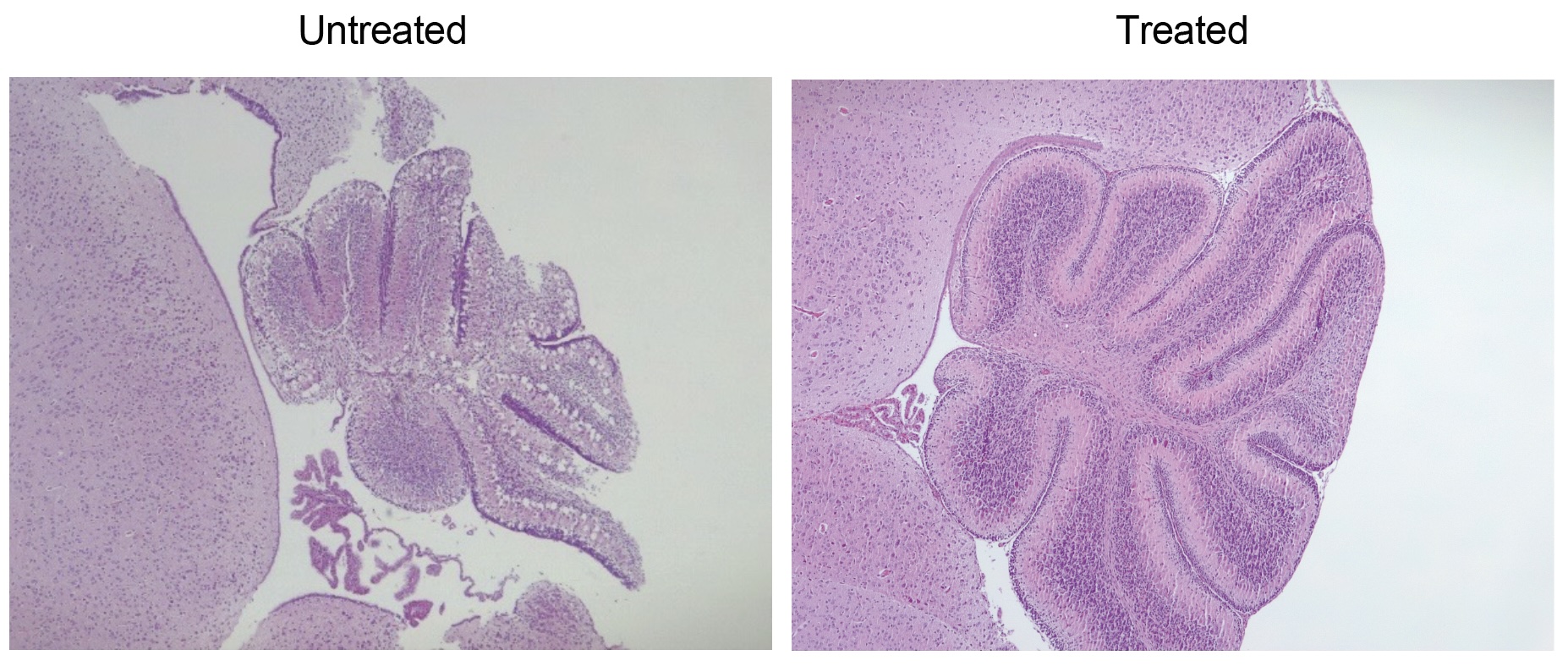
They tested their ideas in genetically modified lab mice that do not create HPDL. At baseline, these mice develop severe seizures and die within 15 days of birth. Mice verbally treat with COQ10 does not store them.
Instead, the team tried to give the mice to 4-HMA and 4-HB, so they essentially bypassed the missing dominoes in the chain. If treated well early after birth, 90% of mice given this treatment showed limited neurological symptoms (such as foot weakness) of normal lab mice over 18 months.
“His data was very persuasive for me,” Miller said of Pacordo’s discovery. Looking at the benefits of mouse treatment and the lack of side effects in mice, Miller agreed that the same molecule could have been referred to a clinic by someone with HPDL deficiency, that is, an 8-year-old patient.
“The risk of trying this seems to have been low, although in a way it could not be quantified,” she said. “And the benefits are probably huge and there’s a warning that animal models aren’t necessarily well converted to humans.”
Learn more
Like lab mice, 8-year-olds took treatment orally daily in a water-soluble solution. The patient was treated only with 4-HB, as the compound is already available in “very pure form,” the researchers said in the report.
The child says the medicine is sour and colder, Miller pointed out, but overall it’s pretty appealing. Now that he began treatment in December 2023, he can do all his daily functional activities, except sports, himself,” his family told Live Science.
Taken together, studies and test results suggest that treatment can restore function and increase survival in both mice and humans that lack HDPL. SiegfriedHekimi told Live Science in an email that she is a professor in the Department of Biology at McGill University who is not involved in the research.
However, based on available data, Hekimi questions whether the improvements seen in lab mice and 8-year-old boys are directly related to the restored Coq10 levels or other mechanisms. The increase in 4-HMA and 4-HB, independent of CoQ10, is not yet fully understood and may have unique effects that actually explain the therapeutic effects of the medication. At this point, Hekimi argued, it is speculation to suggest that this same treatment could treat other types of COQ10 defects.
The study authors agreed that more research is needed to understand exactly how experimental treatments work.
Pacorde emphasized that this experimental treatment has been tested so far in only one patient. “The next step immediately is to lead this to a patient with this disease and establish whether the reaction they saw in this child is typical,” he said.
But for now, Miller is looking at reasons for hope.
“We all know that research doesn’t always arise as hope, and life doesn’t happen like hope,” Miller said. But “This is a really happy story. It’s a heartwarming story. It’s a child who appears to have been very sick with symptoms in a very short amount of time and he’s getting much better.”
This article is for informational purposes only and is not intended to provide medical advice.
Source link