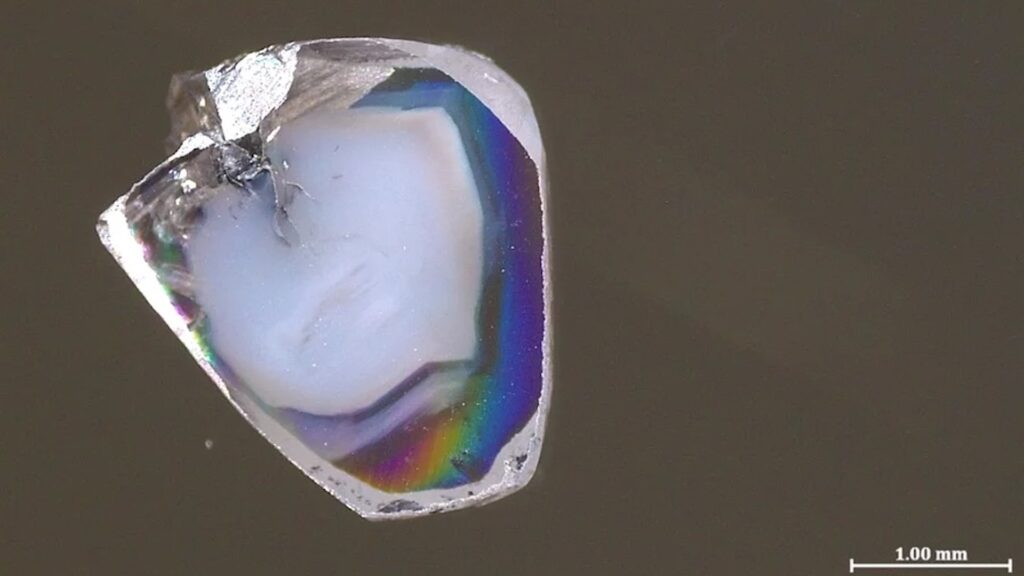
The pair of diamonds, which form hundreds of kilometers deep in the Earth’s adaptive mantle, both have specks of material formed in completely opposed chemical environments. The presence of matter provides a window into the chemical movement of the mantle and the reaction that forms diamond.
Two diamond samples were found at South African mines. Like many other precious gems, they contain what is called inclusions. A small bit of surrounding rocks photographed in diamond form. These inclusions are hated by most jewellers, but they are exciting sources for scientists. This is especially true when diamonds form deep within an unreachable mantle, as they essentially carry these inclusions without being disturbed by the surface.
Two new diamond samples contain inclusions of carbonate minerals rich in oxygen atoms (a state called oxidation) and oxygen-deficient nickel alloys (reduced in chemical terms), respectively. Salt, oxidized carbonate minerals and reduced metals do not coexist for a long time, just like the way acids and bases react quickly to form water and salts. Usually, only one or the other of diamond inclusions are shown, so there are both confused Jaakov Weiss, a senior lecturer in Earth Sciences at Hebrew University in Jerusalem, and his colleagues are initially confused by putting the sample aside for a year.
You might like it
However, when they reanalyzed the diamond, the researchers realized that inclusion captured a snapshot of the reaction that created the sparkling stone, and for the first time confirming that diamond can form when reducing carbonate minerals and metals in the mantle reaction. The new sample is the first time scientists saw the midpoint of that reaction captured in natural diamonds.
“It’s basically two aspects [oxidation] Spectrum said Weiss, senior author of a new study explaining Find, published Monday in Nature Geoscience.
This discovery affects what lies in the mystical middle of the mantle. Moving deeper into the Earth, away from the surface, rocks and minerals become increasingly decreasing and fewer oxygen molecules, but there is little direct evidence of this shift from the mantle.
Theoretical calculations have given the researchers a concept of how planets move from oxidation to depth reduction. “We knew that reduction in some empirical data. The actual sample was reduced to probably 200 kilometres,” says Mayakopirova, professor of Earth, Ocean and Atmospheric Sciences at the University of British Columbia. “What happened under 200 km [was] Our ideas, only our models. Because it’s very difficult to get the ingredients.
These new samples, between 280 and 470 km from the surface of the earth, provide the first real-world fact check of this theoretical mantle chemistry. One finding, according to Weiss, is that oxidized, melted material exists deeper than expected. As kimberlite is an erupted rock that brings diamonds to its surface, it is oxidized, and researchers thought it would not be able to go well below a depth of 300 km. However, these findings suggest that oxidized rocks occur deeper than that, so the kimberlite rocks may be.
Diamond-forming reactions can occur when carbonate liquid is being dragged by a subducted tectonic plate. (Another way that chemists think that diamonds can form is to precipitate from a carbon-rich liquid that cools as it rises up in the mantle, just as sugar crystallizes from the syrup. A new paper does not rule out that process as well.)
Nickel-rich inclusions can also help explain strange occurrences in some diamonds. There is an alternative to the carbon of the carbon in these diamonds. That was a mystery, as nickel is much heavier than carbon, so it shouldn’t be easily replaced with crystal structures, says Kopirova. “Now, looking at these data, we can see that it may be a sign of a diamond layer at a certain depth,” she says. “That would be very interesting to investigate further.”
This article was first published in Scientific American. ©ScientificAmerican.com. Unauthorized reproduction is prohibited. Follow Tiktok and Instagram, X and Facebook.
Source link