simple facts
Milestone: ‘Buckyball’ discovered and described
Date: November 14, 1985
Location: Rice University, Houston, Texas
People: Harry Croteau, Richard Smalley, Robert Carle
In 1985, scientists spent 10 frenetic days devising a new molecule with perfect symmetry, which they named after one of the 20th century’s most famous inventors and futurists.
However, radio and light-based data obtained from this interstellar medium suggested that carbon chains were much longer than expected given the molecular synthesis theory of astrophysics at the time. Scientists began to think that cooling red giant stars might be sending out interstellar medium filled with these six to eight carbon chains.
you may like
Kroto’s serendipitous moment came when he visited the labs of chemists Robert Karl and Richard Smalley at Rice University. Smalley had special equipment in which a laser beam vaporized the atoms on the surface of the metal disk, swept it up into a helium cloud and vacuum to cool it, and finally used another laser to analyze the composition of the atoms.
Kroto began wondering if it would be possible to simulate the outer shell of a cold red giant star by replacing the metal disk with one made of graphite, a type of carbon.
Over the first 10 days of September, the trio, along with graduate students Sean O’Brien and Jim Heath, produced a chain of six to eight carbons that supports the red giant theory.
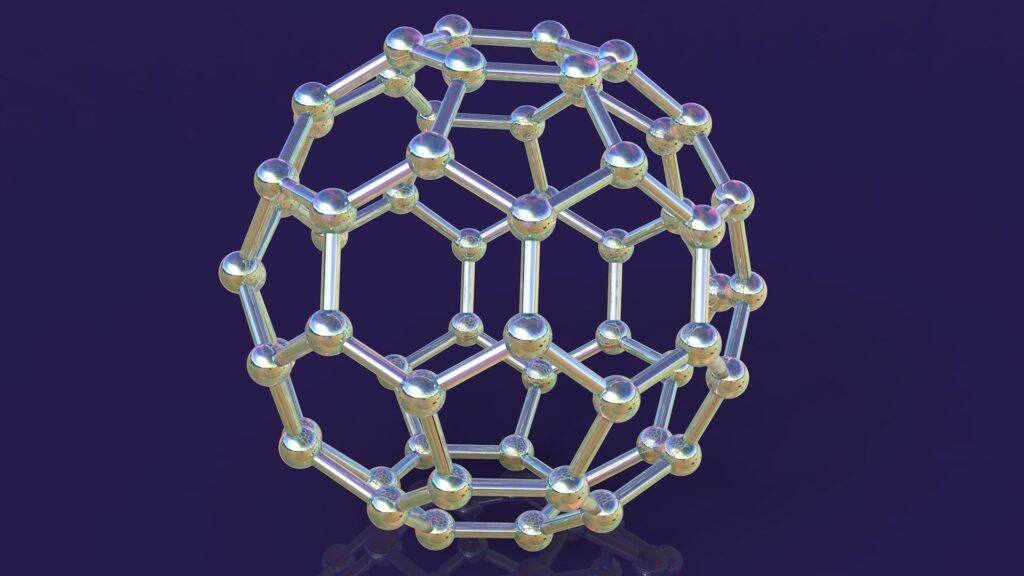
But there were also some intruders. It was a strange form of carbon made up of 60 carbon atoms and a low concentration of an even larger byproduct made up of 70 carbon atoms. These “uninvited guests,” as Kroto called them, had actually been discovered about a year earlier in an experiment at the Exxon Corporate Science Institute in New Jersey, but no one paid much attention to them.
After several days of work, the team reached a conclusion on its structure on September 9th. “C60 actually doesn’t seem to be reactive at all, a behavior that is difficult to reconcile with flat hexagonal graphene sheets, which is the most obvious initial thought,” Kroto said.
In theory, a flat graphene sheet should have a large number of dangling bonds that increase its reactivity.
Scientists spent days trying to figure out the structure of this 60-carbon molecule using toothpicks, jelly beans, paper cutouts of hexagons and pentagons, and other “low-tech” modeling solutions.
you may like
Kroto recalled the 1967 World’s Fair in Montreal. There, 20th century futurist and inventor Buckminster Fuller was exhibiting the geodesic dome, a spherical structure with a network of triangles on its surface that he patented in the 1950s. Smalley went to his office to get a book detailing Fuller’s work, and they understood the proposed structure.

The resulting compound, which they named Buckminsterfullerene, was a molecule with surprising symmetry. A paper describing their new molecules was published in the journal Nature on November 14, 1985, and they soon became known as buckyballs.
Over the next several years, the team deduced the properties of a class of closed molecules called fullerenes. And by 1990, scientists discovered that they could create buckyball chunks by creating an electric arc between two carbon rods.
Croteau, Smalley, and Carle were awarded the 1996 Nobel Prize in Chemistry for their discovery and characterization of buckyballs.
A type of fullerene has proven useful, and nanotubes, a buckyball-like chemical, are extremely strong and have high thermal and electrical conductivity. These nanotubes are important in atomic force microscopy, batteries, coatings, and biosensors. But while scientists have proposed uses for buckyballs in everything from quantum computing to drug delivery, they have yet to find a niche in mainstream applications.
Source link