Mitochondria are primarily known as energy-producing components of cells. However, scientists are increasingly discovering that these tiny organelles do more than just a power cell. They are also involved in immune functions such as controlling inflammation, regulating cell death, and responding to infection.
Research from my colleagues and I have revealed that mitochondria play another important role in your immune response: it senses bacterial activity and helps in neutrophils, a type of white blood cell, trapping and killing.
Over the past 16 years, my research has focused on understanding the decisions that immune cells make during infection and how breakdowns in these decision-making processes can cause illness. A recent discovery in my lab revealed why people with autoimmune diseases such as lupus struggle to fight infections and reveal a potential link between dysfunctional mitochondria and weakening of immune defenses.
You might like it
Secret weapon of the immune system
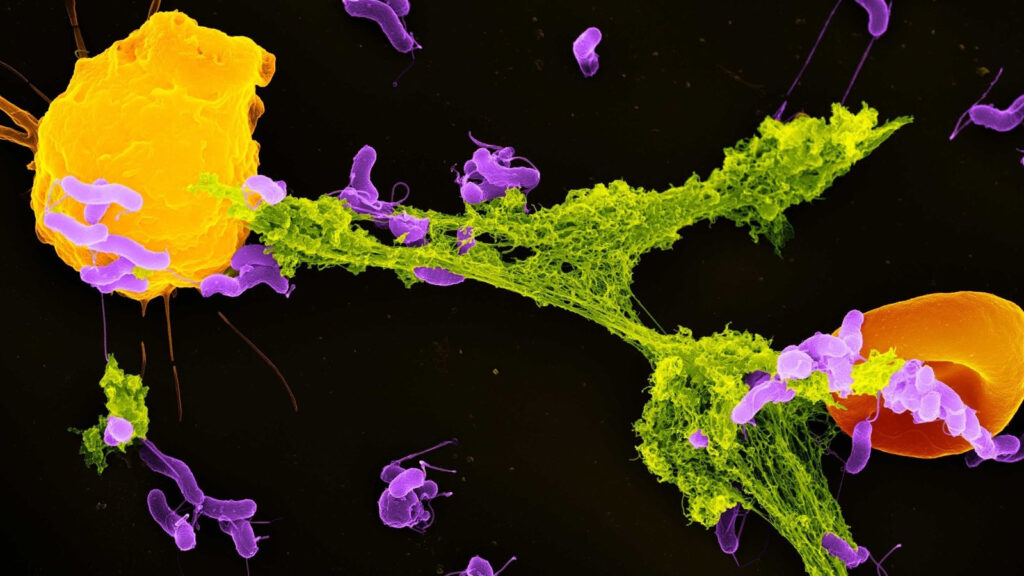
Neutrophils are the most abundant type of immune cells and act as the first responders of the immune system. One of their main defense mechanisms is the release of neutrophil extracellular traps, or nets, which are web-like structures made up of DNA and antimicrobial proteins. These sticky nets trap and neutralize invading microbes, preventing spreading within the body.
Until recently, scientists believed that net formation was primarily caused by cellular stress and damage. However, our study found that mitochondria could detect a specific bacterial by-product of lactic acid and use that signal to initiate net formation.
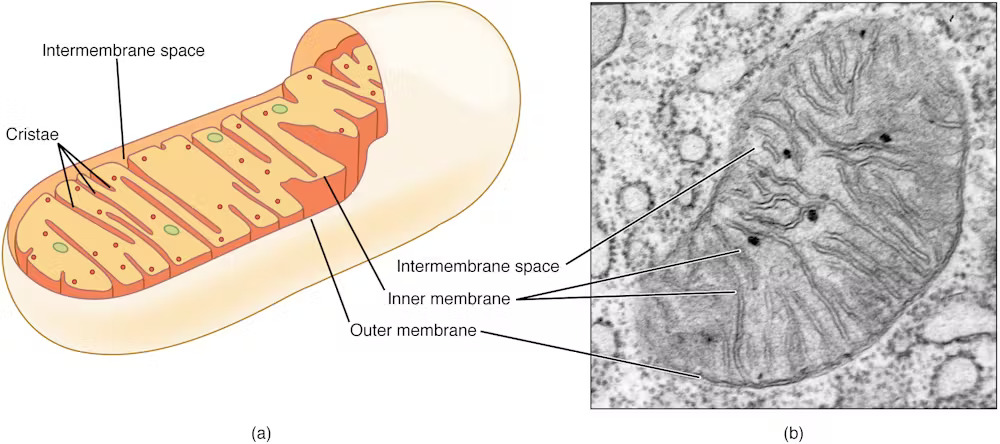
Lactic acid is commonly associated with muscle fatigue in people. However, in the context of bacterial infections, it plays a different role. Many bacteria release lactic acid as part of their own energy production. My team discovered that neutrophils can sense this presence of lactic acid when bacteria are enveloped in a compartment of cells called phagosomes.
Related: 8 babies escaped the potentially fatal genetic disease of the new IVF “Mitochondrial donation”
Within the phagosome, this lactic acid communicates to neutrophils, telling that bacteria are present and that the antibacterial process is not sufficient to kill these pathogens. When neutrophil cell mitochondria detect this lactic acid, the cell begins signaling to remove the trapped web. When cells are released outside the cells, other immune cells can kill them.
Blocking the mitochondria’s ability to sense lactic acid, neutrophils were unable to effectively produce reticulum. This meant that bacteria were likely to escape capture and growth, indicating how important this mechanism was for immune defense. This process highlights a complex dialogue between bacterial metabolism and host cell energy machinery.
What makes this discovery surprising is that intracellular mitochondria can detect bacteria trapped in phagosomes, despite the microorganism being surrounded by another space. Somehow, mitochondrial sensors are able to pick up cues from within these compartments. This is an impressive feat of cell coordination.
Targeting mitochondria to combat infections
Our research is part of a growth field called immune metabolism, and explores how metabolism and immune function are deeply intertwined. Rather than viewing cell metabolism as a strict energy-generating tool, researchers now recognize it as a central factor in immune decisions.
Mitochondria sit at the heart of this interaction. The ability of cells to sense, respond and further shape the metabolic environment gives an important role in determining how and how immune responses are unfolded.
For example, our findings provide the main reason why patients with chronic autoimmune disease called systemic lupus erythematosus often suffer from recurrent infections. Neutrophil mitochondria in lupus patients are unable to properly sense bacterial lactic acid. As a result, net production has declined significantly. This mitochondrial dysfunction explains why lupus patients are more vulnerable to bacterial infections, despite the constant activation of the immune system due to illness.
This observation points to a central role for mitochondria in balancing immune responses. It connects two seemingly unrelated issues: immune debilitating, such as immune overload and increased susceptibility to infection, as seen in lupus. When mitochondria function properly, neutrophils can help to effect effective and target attacks against bacteria. However, when mitochondria are impaired, the system collapses.
Our discovery that mitochondria sense bacterial lactic acid and cause net formation opens up new possibilities for the treatment of infectious diseases. For example, drugs that enhance mitochondrial sensing may boost net production in people with weakened immune systems. Conversely, limiting this response may be beneficial in conditions in which nets contribute to tissue damage (such as severe Covid-19 or autoimmune disease).
Furthermore, our study raises the question of whether other immune cells use similar mechanisms to sense microbial metabolites and whether other bacterial by-products function as immune signals. A more detailed understanding of these pathways can lead to new treatments that regulate the immune response more accurately, reducing collateral damage while maintaining antibacterial protection.
Mitochondria are not just the cellular powerhouse. They are the immune system watchdogs and alert even the weakest metabolic signals of bacterial invaders. As researchers’ understanding of the role expands, so does appreciation for the complexity and adaptability of cell defense.
This edited article will be republished from the conversation under a Creative Commons license. Please read the original article.
Source link