Physicists studied an unusual molecule to determine for the first time how magnetism is distributed within the nucleus of a radioactive atom.
Generally speaking, the laws of nature do not change. If you throw a ball in Seattle or Tokyo, it will fall the same way. Physicists call this “symmetry” and use symmetry as a guide to how the universe should behave. That is what keeps the world consistent. If the laws of physics worked differently on Tuesday, the universe would be in chaos.
But parts of nature don’t seem to follow this perfect balance. For example, it might seem fair to think that the universe should treat matter and antimatter equally. But our universe is made almost entirely of matter, and physicists still don’t know why.
you may like
One promising place to look for answers is inside radioactive atomic nuclei. That’s because the uneven arrangement of protons and neutrons can magnify even the slightest symmetry break. If scientists can detect these tiny asymmetries, they could reveal new physics beyond the Standard Model, said Silviu Marian Udrescu, an MIT physicist and co-author of a new study on the phenomenon.
In a study published October 23 in the journal Science, scientists at CERN and MIT examined a short-lived radioactive molecule called radium monofluoride (RaF) and measured its energy spectrum. But surprisingly, they got to observe for the first time how magnetism is distributed within one of the nuclei. This phenomenon, known as the Bohr-Weiskopf effect, has never been observed in molecules before.
atomic avocado
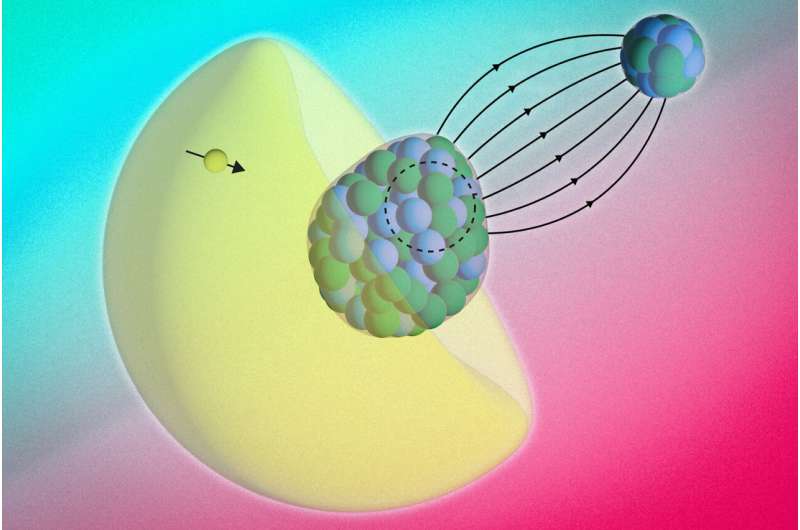
The RaF molecule is made up of two atoms: radium and fluoride. Each has its own core. The radium nucleus has a property called “octupole deformation”.
“You can think of the nucleus itself as pear- or avocado-shaped,” Shane Wilkins, an MIT physicist and lead author of the study, told Live Science. RaF’s asymmetrical shape makes it the perfect candidate to find the asymmetry the team was looking for.
“This is a very rare property,” Udrescu added. “This occurs in only a small number of nuclei in the entire nuclear diagram. And all of the nuclei that are shaped like this pear are radioactive.”
These isotopes are unstable and short-lived, making it difficult to study the nucleus due to radioactivity. That means they decay within about 15 days, potentially disappearing before researchers can make many measurements. “We can only produce very small quantities,” Wilkins said.
The Bohr-Weiskopf effect has been observed in individual atoms where electrons interact with a single atomic nucleus. However, detecting it in molecules is more difficult. That’s because electrons are constantly moving between the two nuclei. Movement can blur the magnetic signal, making it difficult to detect. In the RaF molecule, the fluoride atom is a simpler binding partner. This allows scientists to focus on the magnetic structure of the heavier radium nucleus.
you may like
The research team first created radium monofluoride at CERN’s ISOLDE facility. They blasted a uranium target with high-energy protons to produce the rare isotope radium-225, which they combined with fluorine gas. Each molecule exists only for a moment. The researchers were only able to detect about 50 particles per second under conditions suitable for measurement.
They then aimed multiple laser beams at slightly different frequencies at the molecules. When molecules absorbed or emitted light, scientists recorded small changes in that light. This produced a spectrum. These patterns typically tell scientists how electrons move around the nucleus of an atom. But in this case, several changes revealed that the electrons were being influenced from within the nucleus.
“Because the electrons actually probe the interior of the atomic nucleus, we can no longer treat them as long-range interactions. Instead, the electrons begin to sense internal properties of the radium nucleus itself,” Wilkins said.
“This effect is called the Bohr-Weiskopf effect,” Wilkins added. “To our knowledge, this effect has never been observed in molecules before. The fact that we were able to observe this effect experimentally and explain it theoretically says a lot about how well-suited these molecules are for future precision measurements.”
Now that the researchers have mapped the internal structure of RaF, they can use it to investigate even smaller effects that can break natural symmetries. Wilkins said the next step is to slow down and capture these molecules with a laser to perform more precise measurements.
“We now know that they can be powerful tools for searching for new physics,” Udrescu said.
Source link

