Professor Soni Savai Pullamsetti from the Restoring Roots project emphasises the importance of addressing pulmonary hypertension and describes how the project will help achieve this.
Pulmonary hypertension is an increasingly recognised, serious condition that affects millions of individuals worldwide, posing challenges not only to patients but also to healthcare systems. Advancing our understanding of its underlying mechanisms can pave the way for innovative therapeutic strategies, and the Restoring Roots project is at the forefront of this endeavour.
Led by the dedicated team at the Pullamsetti Lab within Justus Liebig University, the Restoring Roots project investigates the relationships between epigenetic factors and transcriptional regulation in the development of pulmonary hypertension. To understand more about the condition and how the project hopes to provide a solution, The Innovation Platform spoke with Professor Soni Savai Pullamsetti.
For those unfamiliar, what is pulmonary hypertension, and how does it affect people living with the disease?
Pulmonary hypertension is a disease that affects both the heart and lungs. Initially, the blood vessels in the lungs become damaged; they undergo changes that cause them to stiffen and narrow. The stiffening occurs due to cell proliferation that leads to vessel narrowing, a process known as remodelling. This results in increased workload on the right ventricle of the heart, which ultimately can cause heart failure.
Patients with pulmonary hypertension typically feel very fatigued, often suffering from dyspnoea, or shortness of breath, making even simple tasks incredibly challenging. Unfortunately, the five-year survival rate for pulmonary hypertension is still quite low, and despite some advancements, the primary bottleneck remains in improving the quality of life for these patients.
lab (Priyasha De)
Once considered a rare disease, we now see the disease appearing globally. The causes vary by region: In India, we see many children developing pulmonary hypertension from congenital heart diseases. In Africa, conditions like schistosomiasis appear to be a contributing factor, and in places like China, high altitude causes increased susceptibility. These various forms of pulmonary hypertension now suggest that up to 100 million people worldwide are affected.
While we do have medications available, they are specifically designed for pulmonary arterial hypertension, which represents only one subset of pulmonary hypertension. Other forms of the disease can arise from a variety of causes – for example, from left heart disease, chronic lung conditions like COPD or pulmonary fibrosis, or even from unresolved blood clots. Unfortunately, for these patients, treatment options remain very limited. This means that while some individuals benefit from targeted therapies, the majority of patients with pulmonary hypertension still face significant unmet medical needs, particularly those whose condition is linked to underlying heart or lung disease.
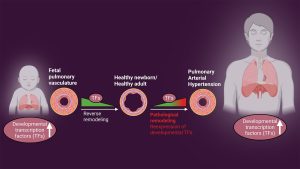
Your project explores how damaged blood vessels start behaving like they did during early development. What led you to this idea, and why is it such an important avenue to explore?
Our lab focuses on how transcription factors and epigenetic mechanisms drive pulmonary hypertension and other chronic lung diseases. Lung diseases aren’t explained by genetics alone – environmental exposures, and how long someone is exposed to them, play a major role through epigenetic changes.
When we analysed the epigenome and transcriptome, we found something striking: about 35% of the regulated genes were tied to developmental programmes and developmental transcription factors. These transcription factors should normally be silent after birth, but we saw them reactivated. That raised the big question – is this reactivation a protective response, or is it contributing to disease?
This led us to think: perhaps we can learn from nature. Blood vessels in early development are remarkably good at growing and adapting. When those same developmental pathways are reactivated later in life, they may drive vascular remodelling — but if we understand them better, we might be able to reprogramme them toward reverse remodelling and regeneration. By identifying the epigenetic mechanisms at play, our goal is to modulate them — not only to halt disease progression but also to restore a healthy vascular architecture.
Another important aspect is timing. The effectiveness of an intervention can vary depending on when it’s given in life. That’s why we want to understand these changes across different stages and tailor therapies accordingly. Clinically, most patients today are staged using imaging or invasive procedures. Our aim is to go further – by integrating omics into practice, we can precisely phenotype patients and move closer to truly personalised medicine.
Restoring Roots draws on some powerful lab techniques and model systems. Could you tell us a bit about the expertise in your team and the kind of work your lab is best known for?
The Restoring Roots project relies on powerful lab techniques and model systems, with a primary focus on lung and vascular epigenetics. During the Restoring Roots project, we concentrated on two primary aspects. First, we conducted extensive omics analyses, including next-generation sequencing, CRISPR-Cas9-based genome editing, epigenetic profiling, and multi-layered epigenomic analysis.
The second aspect is aimed at understanding diseases for both research and therapeutic purposes, developing models that enhance in vivo studies or may even complement or replace them in the future.
One significant model we’ve established is human living lung slices, which involves obtaining transplanted lungs from patients, cutting them into thick sections, and then culturing them for up to 14 days. We can administer specific treatments every few days to observe the response, which is particularly valuable because these transplanted lungs are from patients who have not responded to existing therapies. Through this model, we can evaluate whether new drugs or other molecular interventions are effective.
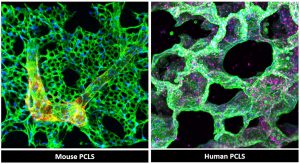
(PCLS), fixed and stained for alveolar and vascular networks. Figure support: Pullamsetti lab (Anoop
Vadakan Cherian)
Our lab is also working on establishing vascular organoid models. These vascularised lung organoids, derived from induced pluripotent stem cells, act as mini-organs in a dish, and they can be utilised for research into vascular regeneration and growth.
Two other techniques we are contributing to the field are through the restoration of relevant biological pathways and the development of transgenic mouse models with specific mutations, such as those in the Tbx4 gene. These mutant mouse models closely mimic the phenotypes observed in human patients, particularly in children with Tbx4 mutations. Through this approach, we can better understand the characteristics of these patients and replicate their conditions in a laboratory setting, offering valuable insights beyond traditional in vitro or in vivo studies that allow us to translate our findings from the bench to the bedside.
Has the project revealed any unexpected findings so far, or opened the door to new research directions you didn’t initially anticipate?
Indeed yes. One of our most unexpected findings has been that developmental transcription factors – genes normally active only in early life – play very different roles depending on timing. In the postnatal period, they can lead to insufficient blood vessel growth, while in adulthood, their reactivation drives harmful vessel remodelling. This shifted our focus from simply studying remodelling to also exploring regeneration by reprogramming these developmental pathways.
We published these findings in Science Translational Medicine, where we also showed that epigenetic therapies – drugs that influence how genes are switched on or off without changing DNA itself – could potentially target these factors. The challenge now is to move these therapies toward clinical trials, while ensuring they reach the right lung cells safely and effectively.
To do this, we are working on precision reprogramming approaches. For example, we have promising data using CRISPR-Cas9 gene editing to control gene expression through enhancers, which are stretches of DNA that regulate how genes work. By identifying lung-specific enhancers, we hope to target the lungs, their blood vessels, and even specific disease-driving cell types with much greater precision.
Beyond new therapies, this project also opens the door to broader applications of ‘omics’ technologies – large-scale analyses of genes and molecular profiles. Pulmonary hypertension begins in the lungs but often causes right heart failure, the main reason patients die. By applying omics to study the right ventricle, we can share valuable datasets with the global community and better understand how disease progresses. This approach is already revolutionising cancer care through theragnostics – combining diagnostics and targeted therapies to match treatment to each patient’s molecular profile rather than just tumour type. Together with Professor Ardeschir Ghofrani, we see the same potential for lung disease: identifying which patients will respond to therapy and tailoring treatments to them more precisely.
Finally, our findings are pushing us to look at environmental factors. Nanoplastics and microplastics, for instance, can accumulate in the body and trap pollutants like smoke in the lungs. This creates a kind of ‘memory’ in cells that may leave long-lasting marks on gene regulation, contributing to chronic disease later in life. Understanding these environmental-epigenetic interactions is an exciting new direction for us.
You’re based across two major research hubs – Giessen and the Max Planck Institute. How do those environments shape your work and connect you to wider scientific networks?
Being based across Giessen and the Max Planck Institute gives our work a unique advantage. At the Max Planck Institute for Heart and Lung Research, we have access to cutting-edge technologies, particularly for omics and epigenomic analysis. At Justus Liebig University in Giessen, we are closer to the clinic, providing direct access to patients, including those with specific mutations. This connection allows us to obtain patient samples and observe in real time how gene expression changes in isolated cells.

Beyond these two hubs, we’re deeply embedded in broader networks. Our lab collaborates internationally with colleagues in the US, Europe, Canada, and India, and locally through the Institute for Lung Health (ILH), the German Center for Lung Research (DZL), and the Cardio-Pulmonary Institute (CPI). These networks provide not only advanced tools and expertise but also an exchange of ideas with outstanding researchers.
More recently, I became an honourable member of the German National Academy of Sciences, Leopoldina. That has opened a platform to contribute to policymaking in areas like genetic engineering and precision medicine, further connecting our research to patients, which is ultimately the goal.
With support from the European Research Council, what comes next? Are you looking to build new partnerships or expand on this work in future calls or consortia?
The European Research Council is unique in that it supports high-risk, high-gain projects — and that is exactly where our work fits. With this support, we are now applying for Proof of Concept grants to bridge fundamental discoveries on disease mechanisms with patient-oriented clinical applications.
One exciting collaboration is with Tbx4 Life, an organisation founded by a father whose child suffers from pulmonary hypertension due to Tbx4 mutations. Together, we aim to develop targeted therapies, improve detection methods, and deepen our understanding of the underlying biology.
Looking ahead, our focus is shifting toward lung remodelling and regeneration. Under the Horizon Europe framework, we are preparing proposals centred on developmental reprogramming and lung regeneration. These efforts will connect us with global experts in the US, Europe, Canada, and the biotech sector, particularly in biomedical engineering. For example, we’ve (Institute for Lung Health) partnered with the Indian Institute of Technology in Hyderabad to establish a specialised lab that can replicate complex lung conditions, including stiffness, perfusion, and oxygenation, to better model disease. This initiative is being led together with Professor Werner Seeger, Professor Rajkumar Savai, and myself.
At the same time, we are initiating early-phase clinical studies on epigenetic therapies. A key focus will be precision medicine, with strategies tailored to target specific organs and disease-driving cell types. Ultimately, our goal is to translate these discoveries into therapies that directly benefit patients, especially in settings where the medical need is greatest.
Please note, this article will also appear in the 23rd edition of our quarterly publication.
Source link