In the film, people are stepped down into ghostly walls. Consider “The Avengers” or Harry Potter going through platform 9¾. It looks easy. But in the real world, if you try that trick, you’ll end up with a nose and many questions.
For example, one question is, why can’t you walk along the wall? The atoms, which are components of matter, are mostly empty spaces. The tiny nucleus about 100,000 times smaller than the entire atoms are located in the center, with electrons located while they are far apart. So why do solid objects feel so…? Do they feel so clearly?
Experts have two physics concepts that make robust materials impossible. The principles of electrostatic repulsion and Pauli exclusion were spoken to Live Science.
You might like it
Classically, atoms have nuclei, made up of protons and neutrons, and there are moving electrons around them. The positive charge of the protons and the negative charge of the electrons pull towards each other and hold the atoms together.
However, in quantum mechanics, electrons do not move in neat circles. Instead, it forms a kind of cloud, that is, an ambiguous area that may be. This is called the “probability cloud,” Raheem Hashmani, a doctoral student in physics at the University of Wisconsin Madison University, told Live Science. This cloud does not work. It just sits there and shows where the electrons are most likely to be seen.
Clouds negatively charge the outskirts of atoms. “When I try to go through the wall, the atoms in my body are [ones] “In the wall, they’re trying to fight each other out,” University of Maryland physicist Stephen Rollston told Live Science.
Related: How many atoms are there in the observable universe?
This is called electromagnetic repulsion, like when you try to push the same poles of two magnets together. When walking along a wall, electrons interact through electromagnetic waves. These waves are part of the force that prevents atoms from overlapping, why do solid matter exist and feel solid?
But what happens if the atom gets even closer?
That is where the principle of exclusion of Pauli appears. Certain particles, known as fermions, say they cannot share the same energy state or be in the same place at the same time. Since electrons are fermions, the terms are interchangeable in this case.
“When these electron clouds begin to approach each other, they overlap. That is, it is possible that the two electrons share the same physical space,” Hashmani explained. “Under Pauli’s exclusion principle, this is not permitted.”
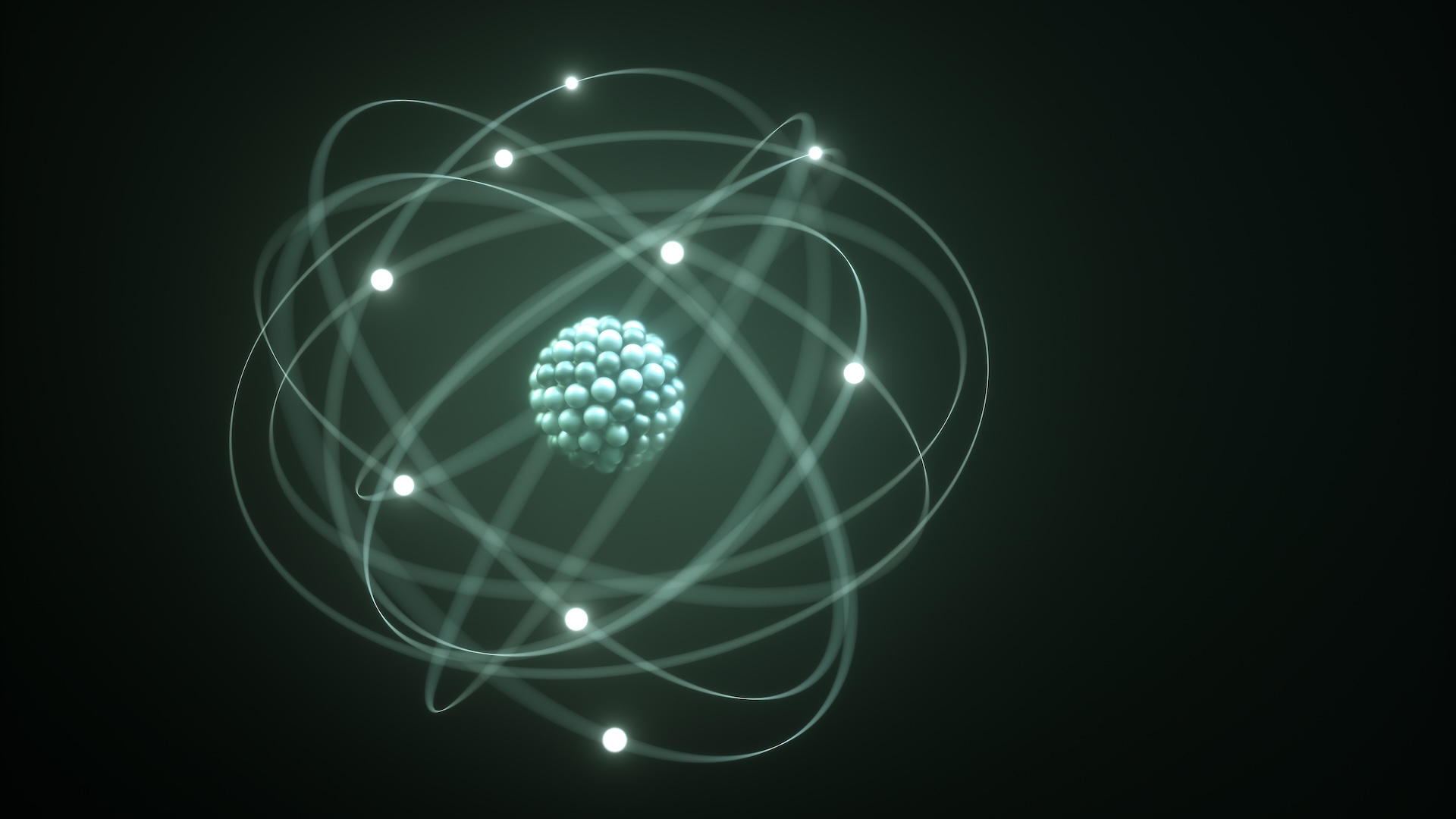
Both concepts (Paulian exclusion principle and electromagnetic repulsion) prevent atoms from occupying the same space. Without them, we know it won’t retain its shape, so it’s a solid thing. In liquids and gases, atoms have more freedom to move, but the same rules still apply. They just make sure the atoms don’t move around, they don’t overlap.
However, even if it is nearly impossible for objects to pass through each other, quantum mechanics always provides an interesting answer. Technically, there’s a small chance that it will happen.
Electron-like particles do not behave like small solid balls. Instead, they behave like waves, and those waves can sometimes stretch past physical barriers.
Let’s say a wave representing a particle hits a wall. With classic mechanics, it would just bounce back. But in quantum mechanics, the waves don’t stop suddenly, Hashmani said. Instead, it starts to collapse exponentially when you enter a barrier. If the wall is thin enough, the waves may still have a small presence on the other side. And since the waves represent the possibility of where the particles are, there is a slight chance that the particles will appear on the other side. This is called quantum tunneling.
Still, the probability of a person passing through a wall is “if it’s something like 1 in 10, it’s 10 power to 30 power,” Hashmani said. “If you put it in a calculator, you effectively give it zero. The calculator on a planet will not give anything that is not zero.
Rolston agreed. “It’s as close to zero as you can get, but it’s not zero,” he said. “It’s so infinitely small and I’m sure it won’t happen in the age of space.”
Albert Einstein Quiz: What do you know about the life of a famous theoretical physicist?
Source link

