Researchers at the Korea Institute of Science and Technology (KIST) have developed a highly efficient carbon catalyst that can effectively produce green hydrogen peroxide.
Importantly, by introducing mesopores into the carbon catalyst, it also functions in air supply environments with low oxygen concentrations and neutral electrolytes.
This breakthrough overcomes the tricky limitations of green hydrogen production, often being limited by the high cost of injecting high purity oxygen gas.
The difficulty of hydrogen peroxide production
Hydrogen peroxide is one of the world’s top 100 industrial chemicals with a wide range of applications in the chemical, medical and semiconductor industries.
Currently, hydrogen peroxide is mainly produced through anthraquinone processes, but this process suffers from several issues, including high energy consumption, the use of expensive palladium catalysts, and environmental pollution due to by-products.
In recent years, environmentally friendly methods of producing hydrogen peroxide through electrochemical reduction in oxygen using inexpensive carbon catalysts have been attracting attention.
However, this method is limited by the high cost of injecting high purity oxygen gas and the practical limitations that the hydrogen peroxide produced is mainly produced in an unstable basic electrolyte environment.
Promotes smooth movement of oxygen
The team synthesized boron-doped carbon containing approximately 20 nanometers of mesopores by reacting greenhouse gas carbon dioxide (CO₂), the powerful reducing agent sodium boron (NABH₄), and mesosized calcium carbonate (CACO₃) particles.
When used as a carbon catalyst for electrochemical hydrogen peroxide production, experiments and calculations have shown that the curved surface properties formed by mesopores provide excellent catalytic activity even in neutral electrolyte environments where hydrogen peroxide production reactions are difficult.
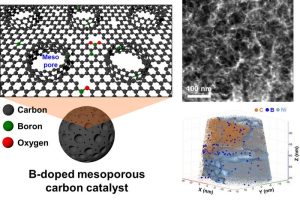
(Left) Schematic diagram of the structure of a porous carbon catalyst in which boron doped on the surface and carbon walls form mesopores.
(Right) Mesoporous structure and atomic scale distribution of boron in carbon catalysts measured using transmission electron and atomic force microscopy.
Furthermore, real-time Raman analysis has confirmed that the mesoporous structure promotes the smooth transfer of oxygen as a reactant, and that it can maintain high catalytic activity even in an air environment with an oxygen concentration of about 20%.
New carbon catalysts are very efficient
Based on these findings, the team demonstrated that boron-doped mesoporous carbon catalysts can achieve world-class hydrogen peroxide production efficiency when applied to a hydrogen peroxide mass production reactor.
These efficiencies can exceed 80% under competitive conditions near neutral electrolyte and air supply and industrial scale current density (200 mA/cm²).
In particular, the team has successfully produced a 3.6% concentration of hydrogen peroxide solution, which exceeds medical hydrogen peroxide concentrations (3%), suggesting the possibility of commercialization.
Dr. Jong Min Kim of Kist said: “Mesoporous carbon catalyst technology, which uses oxygen from breathing air to generate hydrogen peroxide from neutral electrolytes, is more practical than traditional catalysts and speeds up industrialization.”
The future of commercialized carbon catalysts
The impact of this study on future research is important in promoting sustainable and efficient methods for hydrogen peroxide production.
The development of boron-doped mesoporous carbon catalysts that can produce green hydrogen peroxide in an air-supplied, neutral electrolyte environment paves the way for expanding the process, reducing energy consumption and minimizing environmental impact compared to traditional methods.
Future research can focus on further optimizing the catalyst structure and composition to enhance its efficiency, stability, and cost-effectiveness.
Furthermore, investigating the integration of this technology into industrial-scale applications such as large-scale nuclear reactors can help address the challenges of commercial hydrogen peroxide production, leading to more sustainable processes in a variety of industries.
Source link

