Scientists at Goethe University have achieved major breakthroughs in the degradation of PFAS and are offering new hope in the global battle against these lasting “eternal chemicals.”
Their newly developed catalysts decompose PFAS compounds quickly and efficiently without relying on rare or toxic heavy metals.
As concerns about PFA contamination grow around the world, this innovative, low-cost solution could change the way we tackle one of the most stubborn environmental pollutants of the time.
Understanding the PFAS threat
Polyfluorinated and polyfluorinated materials, well known as PFAS, have long been welcomed for their incredible properties.
From non-stick cooking utensils and water leaf-like clothing to firefighting foam and industrial lubricants, these synthetic chemicals can withstand heat, oil, dirt and water like most others. But their strength is also their downfall.
The stability of the same chemicals, which makes PFA extremely convenient, is hardly destroyable even in natural environments.
These so-called eternal chemicals accumulate over time in the soil, water, wildlife, and even the human body. With over 4,700 known variations, PFA raises serious concerns around the world.
Some are linked to cancer, hormonal disruption and other health issues, prompting an increasing urgency to develop safe and effective ways to break down PFA.
A new era for the deterioration of PFA
In an important scientific breakthrough, chemists at Goethe University Frankfurt have unveiled a new catalyst that can efficiently decompose PFAS compounds without the need for toxic heavy metals.
Unlike traditional methods that rely on rare and expensive elements such as platinum and palladium, this new approach uses a cost-effective, environmentally safe boron-based structure.
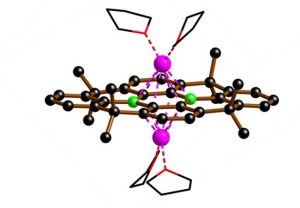
The core of innovation lies in a carbon framework that houses two boron atoms. This unique configuration not only provides the electrons needed to cleave the infamous stable carbon fluorin (C–F) bonds that define PFAS molecules, but also remains stable in air and moisture, a rare property of boron compounds.
Surprisingly, this process occurs at room temperature for just a few seconds and dramatically reduces the energy requirements and complexity of decomposition of PFA.
Towards environmentally friendly chemistry
Today, the catalyst uses alkali metals such as lithium to donate electrons during the reaction.
However, researchers are actively investigating the transition to direct currents as electron sources.
This shift marks a major step forward in making PFA degradation more scalable, but also compatible with green energy sources.
Switching from reactive chemicals to electrical inputs allows you to streamline industrial applications and open doors for more widespread use in environmental restoration technologies.
This is a promising development that blends well with global goals on sustainability and chemical waste reduction.
Possibility of drug integration
The immediate focus lies in degradation of PFA, but the usefulness of the catalyst does not end there. Fluorine atoms are frequently used in pharmaceuticals to increase drug stability and bioavailability.
This new catalyst can provide scientists with unprecedented control over fluorination in drug design, allowing for more accurate synthesis of drugs with improved efficacy and reduced side effects.
Such dual purpose functions underscore the broader importance of breakthroughs. This is highlighted not only as a solution to pressing environmental challenges, but also as a tool to promote future innovation in health and chemistry.
Steps near the end of PFAS legacy
As PFA contamination is increasingly affecting communities and ecosystems around the world, the need for effective, scalable, and non-toxic degradation methods is urgent.
The catalyst developed at Goethe University represents a promising step in that direction, providing a viable way to dismantle these persistent contaminants while avoiding the use of harmful heavy metals.
As research continues, this finding could pave the way for safer environments and cleaner technologies, turning the tide into decades of accumulation of PFA. In the fight against Forever Chemicals, this innovation may prove to be a game changer.
Source link

